-
Call Now
1800-102-2727
Fuel Cells: Definition, Types, Hydrogen-Oxygen fuel cell- Working Principle and Function, Application of fuel cell, Advantages and Disadvantages, Practice Problems and FAQs:
Increase in hike in fossil fuel prices has become a regular affair to the inconvenience of not only public but also a burden on the Government exchequer of foreign reserves. This is because of the limited availability and not being able to match the energy demand of the world. After a couple of decades, the prices of petrol and diesel will become more than gold and in the future, you will see them only in museums.
Research on alternative energy sources, that will be natural, renewable, and non-polluting has been under process. Solar energy utilization bridges the requirement a bit.
The evolution of electric vehicles has just started. What kind of vehicle does your family use in day-to-day life petrol/diesel/electric?
Well, if you use electric vehicles then do you know what kind of batteries they use? Is it eco-friendly?
Electric vehicle
First of all the batteries are generally Lithium-ion batteries which are absolutely difficult to dispose.. Secondly, these batteries need the same electricity produced by burning coals.
Is there any other clean energy source for our vehicles?
Yes. One of the promising energy resources meeting all the criteria is fuel cell. Fuel cells may find applications in space programs, military operations, and many more.
One of the most promising fuel cell is Hydrogen-Oxygen fuel cell.
I hear your question that if it is possible then why we haven’t used it yet?
Come learn about the fuel cell, its promises, and problems to find an answer..
- Definition of fuel cells
- Type of fuel cell
- Working principal of Hydrogen-Oxygen fuel cell
- Advantages of fuel cells.
- Efficiency of the fuel cell
- Difficulties in the construction of fuel cell
- Practice problems
- Frequently Asked Questions (FAQs)
Definition of fuel cells
A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction.
A fuel cell is an electrochemical device that uses an electrochemical reaction to produce electrical energy from fuel. These cells require a continuous input of fuel and an oxidizing agent (generally oxygen) in order to sustain the reactions that generate electricity. Therefore, these cells can constantly generate electricity until the supply of fuel and oxygen is cut off.
These cells need a constant supply of fuel and an oxidising agent (typically oxygen) to continue the chemical reaction that generates electricity. So long as fuel and oxygen are available, these cells can continue to produce electricity. Once the supply of fuel is stopped, chemical reactions will discontinue and so is the generation of electricity.
Type of fuel cells
Fuel cells are classified on the basis of their working chemicals involved. Some examples are-
- Polymer electrolyte membrane fuel cells. ...
- Direct methanol fuel cells. ...
- Alkaline fuel cells. ...
- Phosphoric acid fuel cells. ...
- Molten carbonate fuel cells. ...
- Solid oxide fuel cells. ...
- Reversible fuel cells.
- Reversible fuel cells.
- Alkaline fuel cells.
- Polymer electrolyte membrane fuel cells.
- Solid oxide fuel cells.
- Direct methanol fuel cells.
- Phosphoric acid fuel cells.
- Molten carbonate fuel cells.
Hydrogen - Oxygen fuel cell
The hydrogen-oxygen fuel cell is one such cell that has seen great success. The above cell was used as the primary source of electrical energy on the apollo moon flights. The weight of the fuel was 200kg which was sufficient for 11 days in space. This is in comparison to the many tonnes that the engine-generator set would have required. The product which was obtained from the fuel cell was water. Astronauts are used to drink these water for hydration.
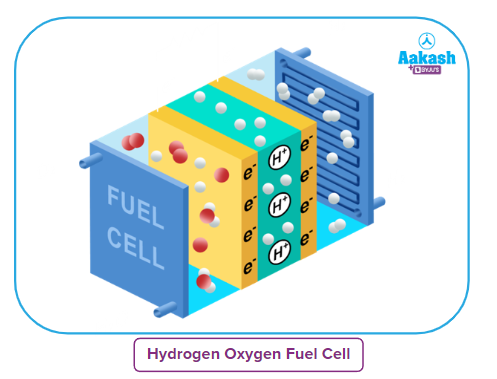
Working principle of Hydrogen-Oxygen fuel cell
The general design of H₂ - O₂ fuel cell is shown in the figure. It consists of porous carbon electrodes containing suitable catalysts (generally finely divided platinum and palladium) incorporated in them.
The figure depicts the basic layout of an H₂ - O₂ fuel cell. It is made up of porous carbon electrodes that are integrated with suitable catalysts, typically finely divided platinum and palladium.
As the electrolyte, a concentrated KOH or NaOH solution is placed between the electrodes. Hydrogen and oxygen gasses are bubbled through the porous electrodes into the KOH/NaOH solution.
A concentrated KOH or NaOH solution is positioned between the electrodes to act as the electrolyte. Gas bubbles of hydrogen and oxygen are introduced into the KOH/NaOH solution through the porous electrodes.
The following electrode reactions take place :
At anode: 2H₂(g) + 4 OH- (aq) → 4H₂O(l) + 4e-
At cathode: O₂(g) + 2H₂O (l) + 4e¯ → 4 OH- (aq)
Overall reaction: 2 H₂(g) + O₂(g) → 2H₂O (l)
Thus, in these cells, the reactants are fed continuously to the electrodes and the products are removed continuously from the electrolyte compartment.
Advantages of Hydrogen-Oxygen fuel cells.
The three main advantages of Hydrogen-oxygen fuel cells are as under:
- Because of the availability and hence continuous supply of hydrogen and oxygen, such cells never become dead. Such a cell is usually operated temperature of 70- 140°C and gives a potential of about 0.9 V.
The theoretical voltage of the cell may be calculated from half-cell reactions as follows:
2 H₂(g) + 4 OH(aq) → 4H₂O (l) + 4e- E0 = + 0.40 V
O₂(g) + 2H₂O (l) + 4e- → 4OH-(aq) E0 = -0.83 V
E0cell = +0.40 V (-0.83 V)= 1.23 V
- Theoretically, the fuel cells are expected to have an efficiency of 100%. However practically they give an efficiency of 60-70 %. Still, they are much superior to the thermal power plants in which fuels are burnt to produce heat which then changes water into steam to run the turbine. Such a power plant does not have an efficiency of more than 40%.
- They do not cause any pollution problems, unlike thermal plants which burn fossil fuels like coal, gas, oil, etc.
Efficiency of the fuel cell
As H is the heat of combustion and G is the useful work done, i.e., the electrical energy produced, therefore, the thermodynamic efficiency () of a fuel cell = ![]() .
.
for the H₂-O₂ fuel cell, theoretically, the value can be calculated as follows:
G =-n FE0cell = -(2) (96500 C mol) (1.23 V) = -237390 CV mol-1=-237.390 kJmol-1
H=285.8 kJ mol-1
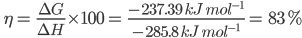
Difficulties in the construction of fuel cell
The construction of fuel cells is, however, faced with certain technical, economic and practical difficulties. The following are some examples: -
- Providing contact between the three phases needed in a fuel cell, i.e., the gaseous fuel, the liquid electrolyte, and the solid catalyst.
- The corrosiveness of the electrolytes used.
- High cost of the catalysts needed for the electrode reactions (Pd,Pt,Ag,etc.)
- Problem of handling gaseous fuels at low temperatures or high pressures.
Applications of fuel cells
Fuel cell technology has numerous applications. Currently, extensive research is being conducted in order to develop a low-cost automobile powered by a fuel cell.
- FCEVs, or fuel cell electric vehicles, use clean fuels and are thus more environmentally friendly than internal combustion engine-powered vehicles.
- They have powered numerous space expeditions, including the Apollo space program.
- Some fuel cells' portability is extremely useful in military applications.
- These electrochemical cells can also power a variety of electronic devices.
Practice problems
Q1. Which of the following statement is correct?
A. Fuel cell converts mechanical energy into electrical energy.
B. Fuel cell converts heat energy into electrical energy.
C. Dry cell is an example of a fuel cell
D. Nicad cell is an example of a fuel cell.
Answer: (B)
Solution: Fuel cells are the devices that convert the energy produced during the combustion of fuels like hydrogen, methanol, and methane directly into electrical energy. Hence, we can say that fuel cells convert heat energy into electrical energy. Dry cell is an example of a primary battery or cell and Nicad is a Nickel-cadmium storage cell is an example of a secondary cell or battery.
Q2. Which of the following solution can be used as an electrolyte during the construction of a Hydrogen- Oxygen fuel cell?
- Conc. NaOH
- Conc. KCl
- Conc. NaCl
- Conc. H2SO4
Answer: (A)
Solution: The electrons supplied by the battery react with the Na+ ions and water in the cathode, forming gaseous (H2) and sodium hydroxide (NaOH). H2 is released into the atmosphere, and NaOH is added to the aqueous solution, raising its pH.
Q3. Which product is released during the combustion of Hydrogen-oxygen fuel cells?
- Salt
- Water
- Hydrogen gas
- Nitrogen gas
Answer: (B)
Solution: During the combustion of the hydrogen-oxygen fuel cell water is collected at the product end. This was useful for the astronauts who used this water for drinking purposes.
Q4. Which of the following is not an advantage of fuel cells?
- Cells never become dead.
- It has an efficiency of 60-70%.
- They don’t cause any pollution problems.
- Construction cost is not very high.
Answer: (D)
Solution: Fuel cells have a lot of advantages such as, due to the continuous supply fuel cells never become dead, theoretically they should have 100% efficiency but as we know no system is practically 100% efficient. Still, fuel cells have 60-70% efficiency. As no fossil fuels are being used for combustion in fuel cells, they are eco-friendly. Apart from all these advantages, one major drawback is that the production cost is very high as they use expensive catalysts.
Q5. What is the thermodynamic efficiency of a hydrogen-oxygen fuel cell?
- 98%
- 52%
- 45%
- 83%
Answer: (D)
Solution: To calculate the thermodynamic efficiency of hydrogen-oxygen fuel cells we have to use the following formula:
thermodynamic efficiency () of a fuel cell ![]()
Where G = Work done during the production of electrical energy
H = Heat of combustion
for the H₂-O₂ fuel cell, theoretically, the value can be calculated as follows:
G =-n FE0cell = -(2) (96500 C mol-1) (1.23 V) = -237390 CV mol-1 = -237.390 kJmol-1
H=285.8 kJmol-1
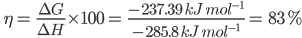
Frequently Asked Questions (FAQs)
Question 1. Do fuel cells require heat to operate?
Answer: Fuel cells function similarly to batteries, but they do not need to be recharged. They generate electricity and heat as long as fuel is available. A fuel cell is made up of two electrodes, one negative (or anode) and one positive (or cathode), sandwiched around an electrolyte.
The two electrodes that make up a fuel cell are sandwiched around an electrolyte and are known as the cathode (positive) and the anode (negative), respectively.
Question 2. How fuel cells are different from batteries?
Answer: In layman's terms, a battery delivers previously stored energy, whereas a fuel cell converts the energy from a fuel to electricity. Electricity is generated by conventional generators as well, but fuel cells generate it directly through a chemical reaction.
Question 3. What is the durability of a fuel cell?
Answer: The fuel cell stacks are intended to last the vehicle's lifetime or approximately 150,000–200,000 miles. At the end of its life, the fuel cell will be disassembled and the materials recycled, similar to how vehicle components are recycled today.
Related Topics
|
Types of Electrodes |
Faraday's Laws |
|---|---|
|
Metallurgy |
Electrolysis |
|
Standard Electrode Potential |
Nernst Equation |
