-
Call Now
1800-102-2727
Sodium Hydrogen Carbonate - Structure, Formula, Preparation, Physical and Chemical Properties, Uses, Practice Problems and FAQ
Who doesn't enjoy eating cakes? Previously, cakes were only associated with birthdays and Christmas! its limitations have since surpassed that. In fact, it's a millennial trend to have a cake-cutting ceremony for all happy occasions, whether it's a wedding, Mother's Day, or Raksha Bandhan, you name it, and cake is an integral part of all such joyous celebrations. Who wouldn't want to sink their teeth into the spongy softness of those delectable layers of fruit cake?
I once asked a baker about the secret ingredient that goes into baking his delightful cakes. Though he had many things to count on, one magical ingredient that rises (pun intended!) the level of his preparation is ‘baking soda’. Well, chemically, that is ‘Sodium Hydrogen carbonate’!
In fact, this chemical does a lot more than just aid in cake baking!
Let's learn more about it, so that the next time you have a cake or bake one yourself, you'll know what you're dealing with.
 TABLE OF CONTENTS
TABLE OF CONTENTS
- Nature and Molecular Formula
- Structure
- Preparation
- Physical Properties
- Chemical Properties
- Difference Between Sodium Carbonate and Sodium Bicarbonate
- Uses
- Practice Problems
- Frequently Asked Questions - FAQ
Nature and Molecular Formula
The molecular formula of sodium hydrogen carbonate is NaHCO3. It is also known as sodium bicarbonate or baking soda. This compound is the monosodium salt of carbonic acid (N2CO3).

Weak Acid Strong Base Salt of (Strong base+Weak acid)
Sodium bicarbonate is alkaline in nature, as it produces a weak acid, H2CO3, and a strong base, NaOH, on hydrolysis. Owing to its alkalinity, it has wide commercial and biological applications.
The naturally occurring mineral form of sodium bicarbonate is nahcolite, and it is found in mineral springs as a component of natron. Sodium bicarbonate (baking soda) is generally confused with sodium carbonate. However, sodium carbonate is washing soda and has its own distinctive properties.
Structure
As the molecular formula NaHCO3 implies, sodium bicarbonate has one sodium, one hydrogen, one carbon, and three oxygen atoms. As sodium carbonate is a monosodium salt of carbonic acid, an electrovalent bond is present between Na+ ion and bicarbonate ion (HCO3). The O - C - O bond present in the structure has a bond angle of 120o, and the molecule has a trigonal planar arrangement.
Sodium hydrogen carbonate has a monoclinic crystalline structure.

Here, an ionic bond is formed between the positively charged Na+ ion and the negatively charged oxygen (which is singly bonded to the central carbon and not bonded to a hydrogen atom).
Preparation
The Solvay process is used for the production of sodium bicarbonate and sodium carbonate industrially.
- Sodium bicarbonate can be synthesized in the laboratory as an intermediate obtained during the Solvay’s process or mined from naturally occurring deposits. However, the percentage obtained from natural ores (nahcolite) is very scarce, so the commercially used method for synthesising sodium bicarbonate is using sodium hydroxide. It is obtained as the intermediate product in the Solvay (ammonia-soda) process.
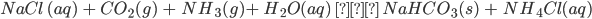
- Sodium bicarbonate can be obtained industrially from sodium carbonate. Carbonate can be changed to bicarbonate by passing CO2 through its saturated solution. NaHCO3 thus obtained is sparingly soluble.
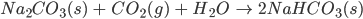
- When carbon dioxide is bubbled through an aqueous solution of sodium hydroxide, sodium carbonate is formed. This sodium carbonate can be converted to sodium hydroxide on further treatment with carbon dioxide.
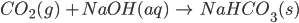
- On average, 100,000 tones/year of sodium bicarbonate is produced from sodium carbonate.
- Nicolas Leblanc, a French chemist, produced sodium carbonate in the year 1791. In the year 1846, Austin Church and John Dwight, bakers of New York, started the first factory to produce baking soda.
Physical Properties
- Sodium bicarbonate is a white crystalline solid with a molecular weight of 84.0066 g mol-1.
- At room temperature, the compound is present in a powdered form with a density of 1.1 to 1.3 g cm-3.
- In its solid form, the density of sodium bicarbonate is 2.20 g cm-3
- The melting of NaHCO3 is 50oC, whereas its boiling point is very high at 851oC.
- The compound is soluble in water, sparingly soluble in methanol and completely immiscible in ethanol.
- On crystallisation, a molecule of sodium bicarbonate forms a monoclinic crystal lattice structure.
- Sodium bicarbonate is an odourless bitter salt.
- Sodium bicarbonate is amphoteric in nature. Furthermore, the refractive index of the molecule is 1.380.
- It is non-flammable and neither its powdered dust is explosive.
- It is an alkaline salt in nature.
Chemical Properties
- It is a white crystalline solid which is sparingly soluble in water. The solution is alkaline in nature due to the formation of hydroxide ions (from NaOH upon hydrolysis.
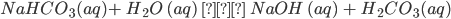
- The aqueous solution of NaHCO3 is weakly basic. It gives a yellow colour with methyl orange but no colour with phenolphthalein.

- On heating, it loses CO2 and H2O, and forms Na2CO3.

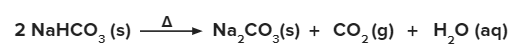
- Sodium bicarbonate, being alkaline in nature, reacts with an acid to form salt and carbonic acid. This carbonic acid later decomposes to form water and carbon dioxide.
For example, Sodium bicarbonate on reaction with acetic acid forms sodium acetate, water and carbon dioxide.

- It reacts with bases like NaOH to produce carbonates.
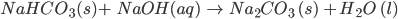
- At temperatures from 80 - 100oC, sodium bicarbonate undergoes decomposition to form sodium carbonate, which gets converted to sodium oxide on further heating in the presence of oxygen.
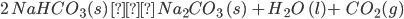
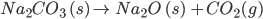
- When kept in the dark and under cold conditions, sodium bicarbonate will not undergo decomposition as it has 2 to 3 years of shelf life.
Difference Between Sodium Carbonate and Sodium Biarbonate
Two commercially useful salts of carbonic acid are sodium carbonate and sodium bicarbonate. Some differences between these two compounds are
|
Sodium Carbonate |
Sodium Biarbonate |
|
Sodium carbonate is also known as washing soda. |
Sodium bicarbonate is also known as baking soda. |
|
Its chemical formula is Na2CO3. |
Its Chemical formula is NaHCO3. |
|
Sodium carbonate is a strong base, and on dissociation, the ions conduct electricity. |
Sodium bicarbonate is a weaker base, this is due to greater stabilisation of carbonate ion due to resonance as compared to bicarbonate ion. |
Uses
Sodium bicarbonate has a spectrum of applications. Some of them are mentioned below.
- It is largely used for making baking powder. Baking powder is a mixture of potassium hydrogen tartrate and NaHCO3.
- It is a mild antiseptic for skin infections.
- Sodium bicarbonate is used as an antacid in the treatment of acidity and chest burns. The molecule of sodium bicarbonate in the body fluids undergoes decomposition to form sodium and bicarbonate ions. These ions act as a buffer and help in neutralising the acidity and thus aid in the treatment.
- Sodium bicarbonate is the major component of dry fire extinguishers. The principle behind the functioning of a fire extinguisher involves producing large quantities of non-combustible carbon dioxide as a by-product of the reaction of sodium bicarbonate and an acid.
- Sodium bicarbonate, colloquially known as baking soda, is used in the baking industry to add fluffiness to the product.
- Sodium bicarbonate is used as a cleaning agent to remove rust and paint from metal surfaces.
- The compound is also added to cattle feed as a buffering agent for the gut of the animal.
- Sodium bicarbonate is effective against microorganisms and hence is used in products of personal hygiene like mouthwashes.
- NaHCO3 is used in reducing the side effects caused due to chemotherapy. This compound is injected intravenously for immediate action.
Practice Problem
Q1. For which of the following diseases, a doctor can prescribe sodium bicarbonate pills?
- Rheumatoid arthritis and/or aching joints
- Migraine
- Shortness of breath
- Acidity
Answer: Sodium bicarbonate is used as an antacid in the treatment of acidity and chest burns. The molecule of sodium bicarbonate in the body fluids undergoes decomposition to form sodium and bicarbonate ions. These ions act as a buffer and help in neutralising the acidity and thus aid in the treatment. So, option D) is the correct answer.
Q2. Name the ore of sodium bicarbonate.
- Nahcolite
- Galena
- Dolomite
- Gypsum
Answer: Nahcolite is the ore of NaHCO3. Galena is a lead ore, dolomite is magnesium and calcium carbonate ore, and gypsum is hydrated calcium sulphate ore. So, option A) is the correct answer.
Q3. What gas is produced when lemon juice reacts with sodium bicarbonate?
- Carbon monoxide
- Carbon dioxide
- Chlorine
- None of the above
Answer: Sodium bicarbonate is slightly alkaline. So, it reacts with an acid to liberate carbon dioxide gas. So, option B) is the correct answer.
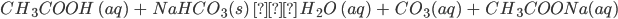
Q4. Sodium bicarbonate has which type of bonding?
- Ionic bond
- Coordinate bond
- Covalent Bond
- Dative Bond
Answer: As sodium bicarbonate is a monosodium salt of carbonic acid, an electrovalent bond is present between Na+ ion and bicarbonate ion (HCO3). So, option A) is the correct answer.
Frequently Asked Questions - FAQ
Question 1. Why is aqueous sodium bicarbonate weakly alkaline?
Answer: It is a white crystalline solid which is sparingly soluble in water. The solution is alkaline in nature due to the formation of hydroxide ions (from NaOH upon hydrolysis.
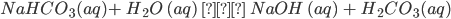
Question 2. Is baking soda and baking powder the same?
Answer: No, baking soda, NaHCO3, is an important active ingredient of baking powder. Baking soda is largely used for making baking powder. Baking powder is a mixture of potassium hydrogen tartrate and NaHCO3.
Question 3. Which is a stronger base, NaHCO3 or NaOH?
Answer: Sodium bicarbonate is a basic salt as it is formed from a weak acid and a strong base. Sodium hydroxide itself is a strong base as it directly undergoes complete dissociation to produce hydroxide ions. So, Sodium hydroxide is definitely a stronger base.
Question 4. Is sodium bicarbonate harmful to human beings?
Answer: It is not generally harmful and also not a potent chemical. But excessive ingestion or inhalation may cause coughing and sneezing. It may also cause gastrointestinal irritation at large.
Related Topics
|
Calcium carbonate |
Sodium Hydroxide |
|
Calcium oxide |
Calcium Sulphate |
|
Sodium Carbonate |
Sodium Chloride |
